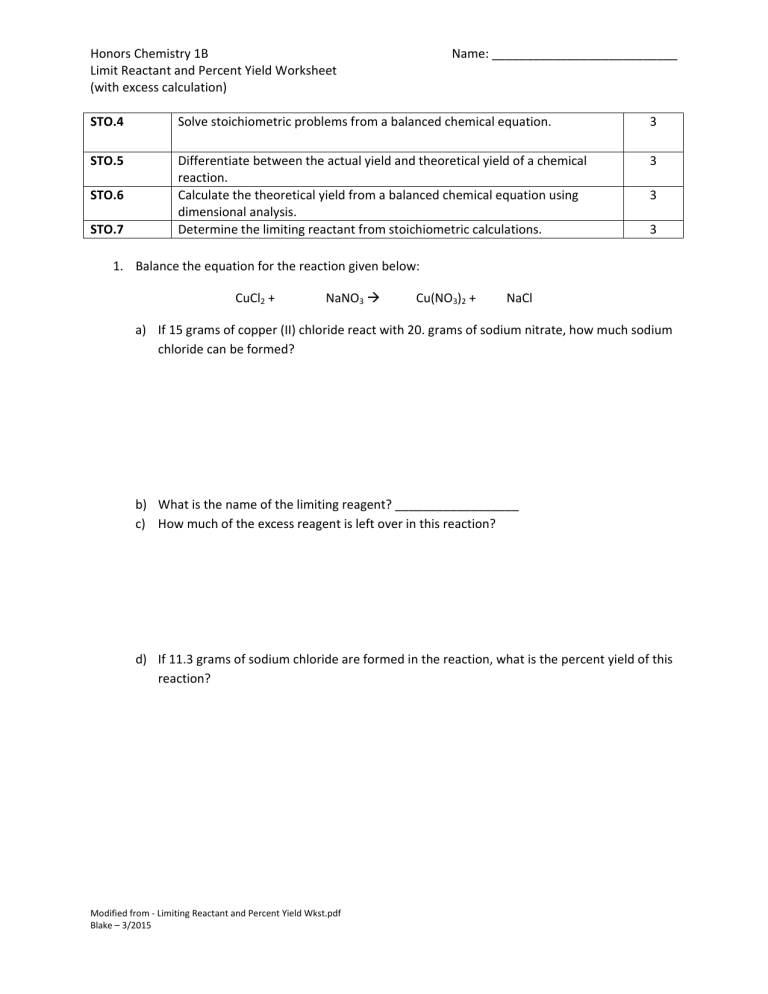
In chemistry, the concept of percent yield and limiting reactants plays a crucial role in determining the efficiency of a chemical reaction. The percent yield is a measure of how much of a desired product is actually obtained compared to the theoretical yield. On the other hand, the limiting reactant is the reactant that is completely consumed in a chemical reaction, thereby determining the maximum amount of product that can be formed.
When conducting experiments in the laboratory, it is important to calculate the percent yield and identify the limiting reactant to optimize the reaction conditions. A worksheet that focuses on these concepts can help students practice their understanding of these fundamental principles in chemistry.
One common type of problem in a percent yield limiting reactant worksheet involves calculating the theoretical yield of a product based on the given reactants and their stoichiometric coefficients. Students are required to identify the limiting reactant by comparing the amounts of each reactant and determining which one will run out first. This information is then used to calculate the maximum amount of product that can be formed.
Another type of problem in the worksheet may require students to calculate the percent yield of a reaction by comparing the actual yield obtained in the laboratory to the theoretical yield calculated from the balanced chemical equation. This allows students to assess the efficiency of the reaction and identify any sources of error that may have contributed to a lower than expected yield.
By practicing with a percent yield limiting reactant worksheet, students can improve their problem-solving skills and gain a deeper understanding of the concepts of percent yield and limiting reactants. These exercises also help students develop critical thinking and analytical skills that are essential for success in chemistry and other scientific disciplines.
In conclusion, a percent yield limiting reactant worksheet is a valuable tool for students to practice and reinforce their understanding of these important concepts in chemistry. By working through various problems and scenarios, students can enhance their knowledge and skills in calculating percent yield and identifying limiting reactants in chemical reactions.